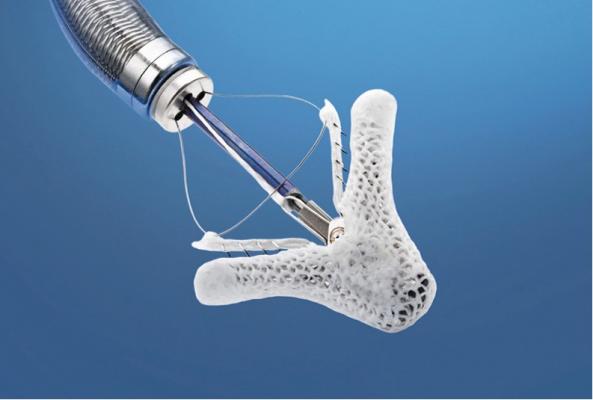
The U.S. Food and Drug Administration has approved a new indication for a heart valve repair device that is intended to reduce moderate-to-severe or severe mitral regurgitation, a leakage of blood backward through the mitral valve into the heart’s left atrium that can cause heart failure symptoms such as shortness of breath, fatigue and swelling in the legs.
When first approved in 2013, the MitraClip Clip Delivery System (MitraClip), manufactured by Abbott Vascular Inc., was indicated to reduce mitral regurgitation in certain patients whose significant mitral regurgitation and heart failure symptoms result from abnormalities of the mitral valve (commonly known as primary or degenerative mitral regurgitation) and whose risks for mitral valve surgery are prohibitive.
The new indication, approved recently, is for treatment of patients with normal mitral valves who develop heart failure symptoms and moderate-to-severe or severe mitral regurgitation because of diminished left heart function (commonly known as secondary or functional mitral regurgitation) despite being treated with optimal medical therapy. Optimal medical therapy includes combinations of different heart failure medications along with, in certain patients, cardiac resynchronization therapy and implantation of cardioverter defibrillators.




