image credit- shutterstock
Australia-based Aspen Pharmacare has announced the Therapeutic Goods Administration (TGA) listing of a new medication to slow the progression of short-sightedness (myopia) in children and young teenagers.
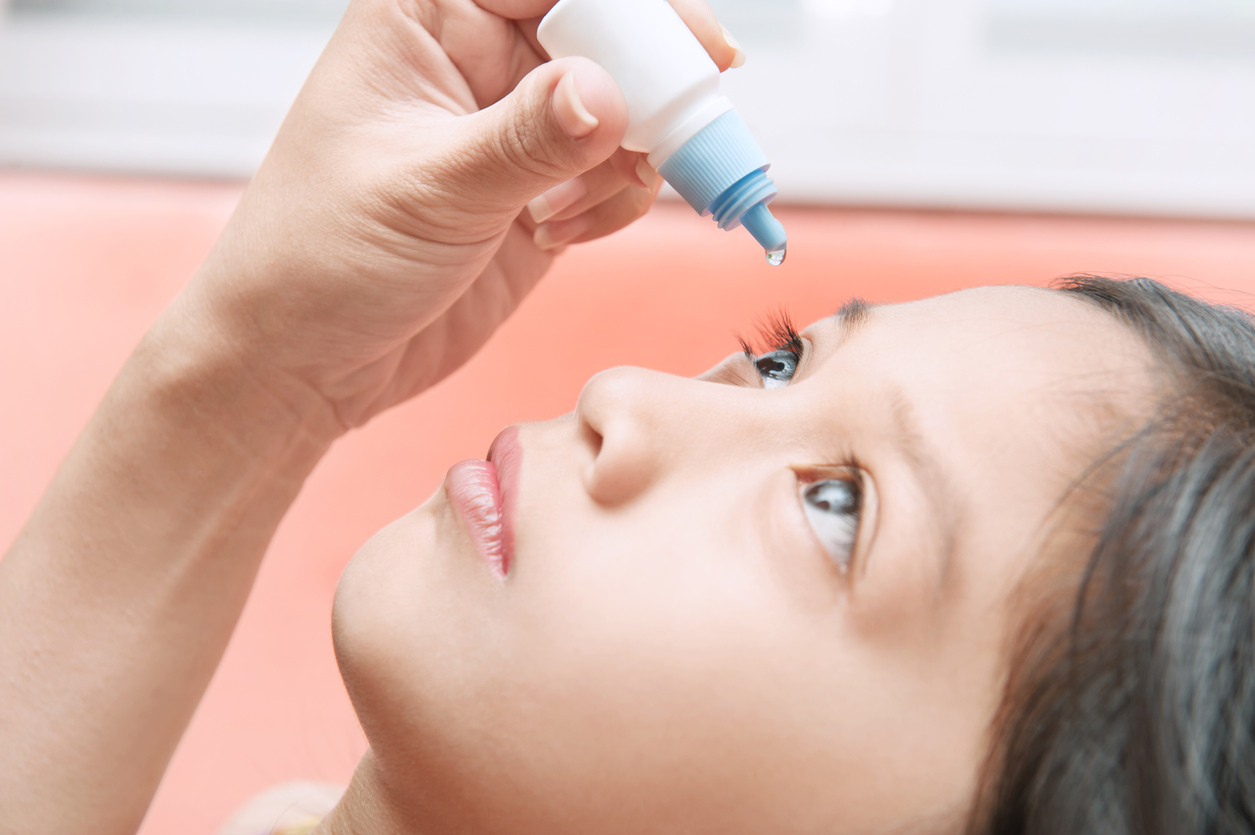
EIKANCE® 0.01% eye drops (atropine sulfate monohydrate 0.01%) are available on prescription for children aged 4 to 14 years, as a treatment to slow the progression of myopia and may be initiated in children when myopia progresses by 1 or more diopters per year.
The TGA registration of EIKANCE 0.01% eye drops was based on several trials including two key randomised, double-blind clinical trials in children with myopia aged 4-12 years and 6-12 years, who were treated with atropine 0.01% eye drops and followed for two to five years to assess the effectiveness and safety of atropine 0.01% eye drops in the treatment of childhood myopia.
There is emerging data indicating that lockdowns during the COVID-19 pandemic may have led to an increase in the incidence of childhood myopia. The likelihood of developing myopia, particularly high myopia, increases when one or both parents have the condition. A survey showed that 91% of Australian parents were not aware of the role excessive screen time could play and 73% did not know that genetics might play a role.




