South Korea-based startup Neurophet, an artificial intelligence (AI) solution company for brain disorders diagnosis and treatment, has announced that its software solution, Neurophet AQUA AD Plus, a comprehensive neuroimaging analysis solution for clinical evaluation related to Alzheimer's disease, has received 510(k) clearance from the US Food and Drug Administration (FDA).
This clearance marks Neurophet's third FDA 510(k) clearance, following Neurophet AQUA, a brain neurodegeneration imaging analysis software, and Neurophet SCALE PET, a PET image quantitative analysis software. The achievement further validates the safety and effectiveness of Neurophet's core product portfolio at a global regulatory standard.
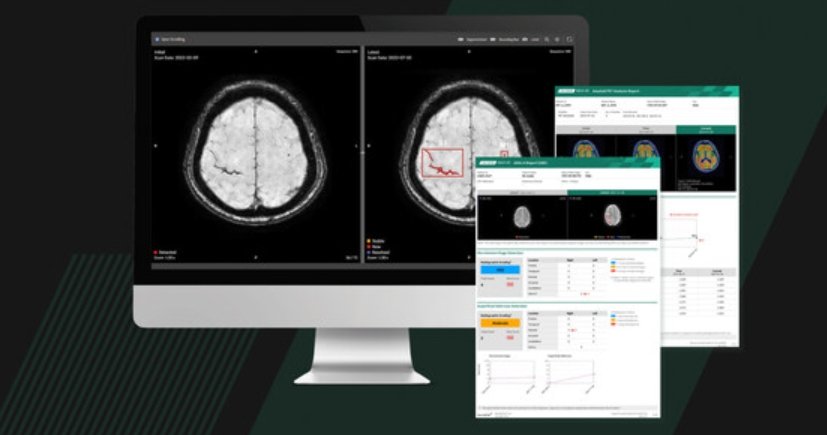
Neurophet AQUA AD Plus is a software-based solution designed to support imaging-based clinical evaluation across the Alzheimer's disease care continuum. The software performs quantitative analysis of MRI and PET images, enabling automated labeling, visualisation, volumetric quantification of brain structures and lesions, as well as standardised uptake value ratio (SUVR) analysis. Quantitative results can be compared with normative reference data to support the evaluation of neurodegeneration and cognitive impairment.
The FDA-cleared US version of Neurophet AQUA AD Plus is an upgraded solution that builds upon the capabilities of Neurophet AQUA AD. Leveraging AI-based brain MRI analysis, the software automatically analyses and quantifies hypointense lesions associated with cerebral microbleeds and superficial siderosis, and hyperintense lesions related to brain edema
By providing automated lesion localisation and counts, the software helps clinicians more precise assess imaging-based risk factors and supports informed, patient-specific clinical decision-making when evaluating patients undergoing Alzheimer's disease-related therapeutic management.




