Lupin achieved 20.4 percent growth in 2011
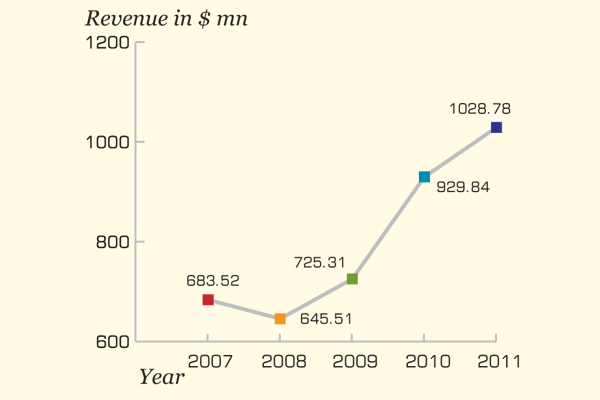
 Lupin Pharmaceutical, which aims to be the best transnational pharmaceutical company in the world, stroked 10.64 percent growth in its sales and marked a successful period in 2011. Lupin achieved double-digit growth in all its businesses, including a robust performance in the US, Europe, India, Japan and South Africa. Lupin also showed impressive growth in international business, achieving 20.4 percent growth in 2011. The total revenue of the firm increased from $929.84 million in 2010 to $1.03 billion in 2011.
Lupin Pharmaceutical, which aims to be the best transnational pharmaceutical company in the world, stroked 10.64 percent growth in its sales and marked a successful period in 2011. Lupin achieved double-digit growth in all its businesses, including a robust performance in the US, Europe, India, Japan and South Africa. Lupin also showed impressive growth in international business, achieving 20.4 percent growth in 2011. The total revenue of the firm increased from $929.84 million in 2010 to $1.03 billion in 2011.
Lupin's formulations business contributed 85 percent to its overall revenues, with the rest coming from active pharmaceutical ingredients (APIs). Lupin has strengthened two factors, its people and research and development (R&D) focus. It invested 8.5 percent of its net sales on R&D. Strengthening its manpower base in the international market, 11 percent of its workforce is based outside India and is spread across over 26 nationalities.
In the US and Europe, it recorded strong performance and the European business reported revenues worth $41 million, registering a growth of 44 percent over last year. The US generics business recorded growth of 39 percent. Out of 30 generic products marketed in the US, 14 are market leaders in terms of market share.
The growth of the company was marked by several drugs getting approved by the Food and Drug Administration (FDA). Lupin received FDA approval for its Fenofibrate tablets, which is a generic equivalent of Abbott Laboratories' Tricor. It also received tentative approval for Eli Lily's generic, Cymbalta. Furthermore, Lupin managed to get an approval stamp for generic Solodyn tablets, generic LoSeasonique tablets and Keppra oral solution. The company also launched authorized generic of Femcon Fe chewable tablets in the US.
Exploring the Japanese market, Lupin Pharmaceutical's Japanese subsidiary, Kyowa Pharmaceutical acquired I'rom Holdings, an integrated Japanese healthcare provider.
Extending its relations with multinational companies, Lupin strategically collaborated with Eli Lilly India to promote and distribute Lilly's Huminsulin range of products.
Lupin is now looking forward to expanding its manufacturing base. It is setting up a new state-of-the-art formulation manufacturing facility at the special economic zone (SEZ), Mihan in Maharashtra, India.
Lupin has 11 world class manufacturing facilities spread across India and Japan, most of which have been scrutinized by leading regulators, such as US FDA, UK MHRA, WHO, Australian TGA, and Japan's MHLW.




