CSL keeps up its innovation strategy
05 June 2012 | Analysis | By BioSpectrum Bureau
CSL keeps up its innovation strategy
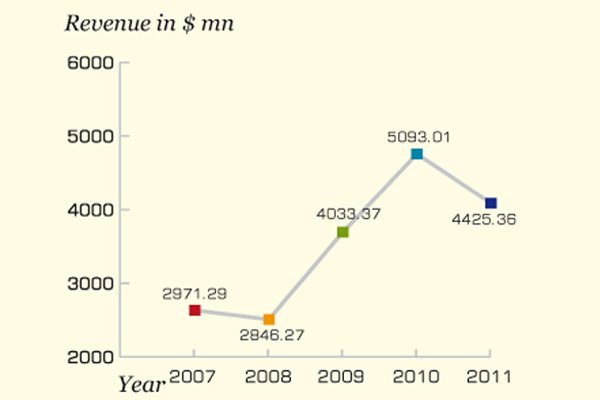
The company witnessed a drop of 13 percent in sales revenue as it stood at $4.43 billion in 2011 against $5.1 billion in 2010
Australia's biggest name in pharmaceutical industry CSL kept its focus on new product development, life-cycle management and safety of its product portfolio in 2011. The company expanded its manufacturing capacity of existing products to meet the rising global demand. The company witnessed a drop of 13 percent in sales revenue as it stood at $4.43 billion in 2011 against $5.1 billion in 2010.
However, CSL's success was driven by its innovative products. Its portfolio of immunoglobulin showed impressive performance and new generation products, Privigen and Hizentra, were approved by multiple regulatory bodies for manufacturing and distribution across the world. Hizentra is the first and only 20 percent subcutaneous immunoglobulin (SCIg) approved in the US that may be stored at room temperature. Subcutaneous immunoglobulin replacement therapy provides patients with the convenience of self-infusion in the comfort of their home. Biostate (human coagulation factor VIII and human von Willebrand factor) sales were also strong, arising from demand for immune tolerance therapy and von Willebrand disease treatments. Expanding and further strengthening its manufacturing capacity is the ongoing strategy of CSL.
Increase in plasma collections by the Australian Red Cross Blood Service and enhancing Australian plasma therapies business has been a key factor in driving CSL's growth. CSL is a global provider of plasma-derived and recombinant products and operates as one of the world's largest plasma collection networks through CSL Plasma. CSL Biotherapies is the chosen national plasma fractionator of Australia, New Zealand, Hong Kong, Malaysia, Singapore, and Taiwan. The giant also signed a worldwide research license and option agreement with Pfizer granting certain rights and options for the use of CSL's Iscomatrix adjuvant.
Defining its strategy for global healthcare, CSL partnered with the world's largest health research agency, the US National Institutes of Health (NIH), to study a potential new treatment for the prevention of congenital cytomegalovirus (CMV) infection, one of the most common known causes of congenital abnormalities in the developed world. Commenting on CSL's outlook for 2012, Dr Brian McNamee, CEO and managing director, CSL, observed, "Looking into fiscal 2012, we anticipate similar trading conditions as in fiscal 2011. Our broad portfolio of products, ongoing product development and our geographic reach position us well to compete effectively."












