(VAI- voluntary action initiated)
Asian biotech industry has been experiencing an escalating boom over the past several years. Pharmaceutical companies have been receiving substantial influx of funds both in the form of FDI and governmental financing programs. China, for instance, being one of the largest pharma economies in the world at a 6.4 per cent growth rate is expected to reach $573.5 billion mark in 2022.
In the meantime, according to the president of South Korea Moon Jae-in’s roadmap, the country’s share of the world pharmaceutical market will reach 6 per cent by 2030, not without the help of government which has allotted a total of $2.5 billion in biotech this year as per the Organisation for Economic Co-operation and Development (OECD).
Given the continuous growth, Asian pharma and biotech companies started looking for more options including new destinations for running multiple clinical trials and obtaining market authorization. According to the state registry of the Ministry of Health of Russia, during the past 5-6 years a certain trend started showing with a growing number of studies initiated by Asian sponsors in Russia.
As per the Russian Ministry of Health Registry of the Approved Clinical Trials, every year, more than 10 per cent of studies initiated by non-local international sponsors in Russia, are accounted for Asian companies. Moreover, throughout the period from 2016 to 2019, more than 250 studies have been conducted by Asian sponsors in the region.
The overall attractiveness of the area to the international sponsors can be confirmed by global statistics as well, with Russia entering the top 10 countries of clinical trials performed in 2018. In addition, clinical trials of 14 out of 158 Food and Drug Administration (FDA) and 48 out of 78 European Medicines Agency (EMA) new drugs were conducted at Russian sites.
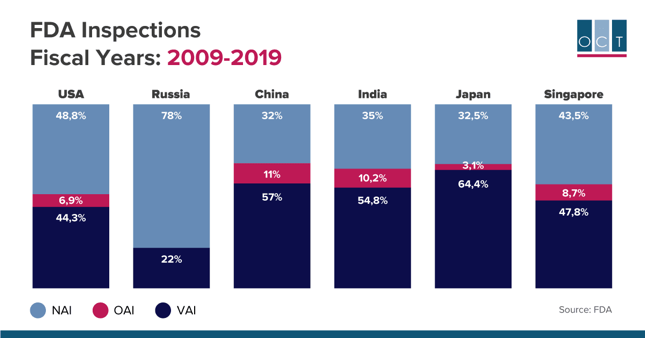
FDA regularly performs inspections of the clinical sites to determine their compliance with applicable regulations and laws. Publicly available statistics of FDA inspections results helps biotech and pharmaceutical companies choose the best options for placing their future clinical studies. During the last 10 years Russia has had the highest NAI rate (No Action Indicated) and has been the only country with no OAI (Official Action Indicated).
According to FDA, Russia comes second after the US in terms of the number of subjects recruited for studies. Through its case log, few leading clinical research organisations in Eastern Europe demonstrate that the enrollment rates in Russia are on average 1.5 times higher than those of the rest of the world.
In addition to the vast pool of patients and record-setting enrollment rates, Asian pharma are looking at the region as a promising marketplace. That explains their interest in market authorization solutions within clinical research services.
One of the attractions of product development in Russia is that Asian companies can carry out high quality and efficient clinical development, taking European business into consideration, in the market where significant growth can be expected.
While patients’ availability and impressive recruitment rates have become one of the trademarks of the Russian clinical research landscape, Asian sponsors have also been closely looking into the full-fledged marketing opportunities in Russia.
Growing demand for market authorization services is further supported by the establishment of the Eurasian Economic Union (EAEU) signed in May 2017, with Russia as one of the member states.
Within the Union with a unified set of regulatory and economic standards and rules, a drug registered in one of the member states can obtain market authorization through a simplified procedure within the rest of the countries of the Union. This development without doubt adds to the appeal of the region among Asian pharma and biotech companies looking for expansion and further growth opportunities.
Irina Petrova, MD, Clinical Operations Director, OCT Clinical CRO, Russia




