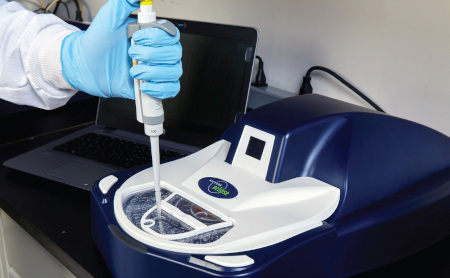
Sievers Eclipse Bacterial Endotoxins Testing (BET) Platform
Since the 1980’s, there have been nominal improvements for most QC labs when it comes to their everyday experiences with compendial endotoxin testing. While automation and robotics were introduced to reduce manual labor and streamline processes, most labs haven’t fully adopted these technologies due to the complexity of the instrumentation, training, and validation practices that come with them. Unfortunately, many automated technologies and new software either don’t meet the needs of users in the QC lab or cannot be implemented and validated without a significant amount of effort. As a result, labs are seeking automated solutions for BET that are easy to implement, fast to validate, and provide day-to-day advantages such as ease of use and decreased contamination.
What is microfluidic automation of BET assays?
Automation using a centripetal microfluidic platform is the simplest form of BET automation available to the market today. It leverages a network of microchannels to direct and mix fluids to automate endotoxin assays. This is achieved within a small, compact microplate that is analyzed using an incubating benchtop spectrophotometer similar in size and function to absorbance microplate readers used for traditional LAL assays.
Centripetal microfluidic automation simplifies endotoxin testing by offering a solution that is extremely easy to set up, use, and maintain. It enables labs to achieve the ease of use, ease of training, and high throughput they want, without having to be concerned about compliance, accuracy, complex validations, or footprint. Using the Sievers Eclipse BET Platform’s microfluidic automation, fully compliant endotoxin assays can be set up in as little as nine minutes with a fraction of the pipetting steps typically required.
By the numbers
With centripetal microfluidic automation using the Sievers Eclipse, fully compliant endotoxin assays are set up in as little as 9 minutes and less than 30 pipetting steps, with up to 21 samples and up to a 5-point standard curve. Just 1 mL of LAL is required.
Spotlight on ease of use
By drastically reducing pipetting steps, centripetal microfluidic automation decreases the complexity of assay setup and mitigates risks and opportunities for errors that lead to costly retests. In addition, microfluidic liquid handling precisely measures all liquids for the end user, which means that the precision typically required during the physical action of pipetting is reduced. With preloaded standards and PPCs, all the technician needs to do is pipette Water for BET, samples, and 1 mL LAL.
Ensuring successful validation of BET platforms
While validation of a new BET platform can be arduous if platforms are complex or suppliers do not provide support for the validation process, there are options available that are streamlined and keep labs functioning at the capacity needed, without disrupting or re-assigning analysts. Simplifying the process allows the QC lab to complete validation in-house or with the help of manufacturer. In an ideal validation scenario, QC labs experience the following:
Validation doesn’t have to be a daunting task. There are options available to QC labs to speed up and simplify the process, ensuring a clear path to success. With an ideal platform, validation can be performed in just a few days, analysts can be fully trained during that time, and system validation is supported by the vendor’s fully documented results for all seven guidelines outlined in USP <1225> and ICH Q2(R1).
Conclusion
There are often many goals associated with moving to an automated BET platform in the QC lab – time savings, simplification, fewer errors, process improvement, improved data management, data integrity, more efficient sign off, and others. Automation of endotoxin testing with microfluidics and the Sievers Eclipse BET Platform offers easier ways of working without compromising on compliance or accuracy. Pipetting, liquid handling, and mixing of reagents are all automated, along with preparation of standard curves and PPCs. Exploiting microfluidics for endotoxin testing reduces consumption of reagents and samples, decreases time setting up reactions, and increases sample throughput. Additionally, with the use of software that is purpose-built for endotoxin automation, QC labs are able to streamline testing, data review, and sign off in ways that meet today’s needs and prepare for the future of pharmaceutical manufacturing.




