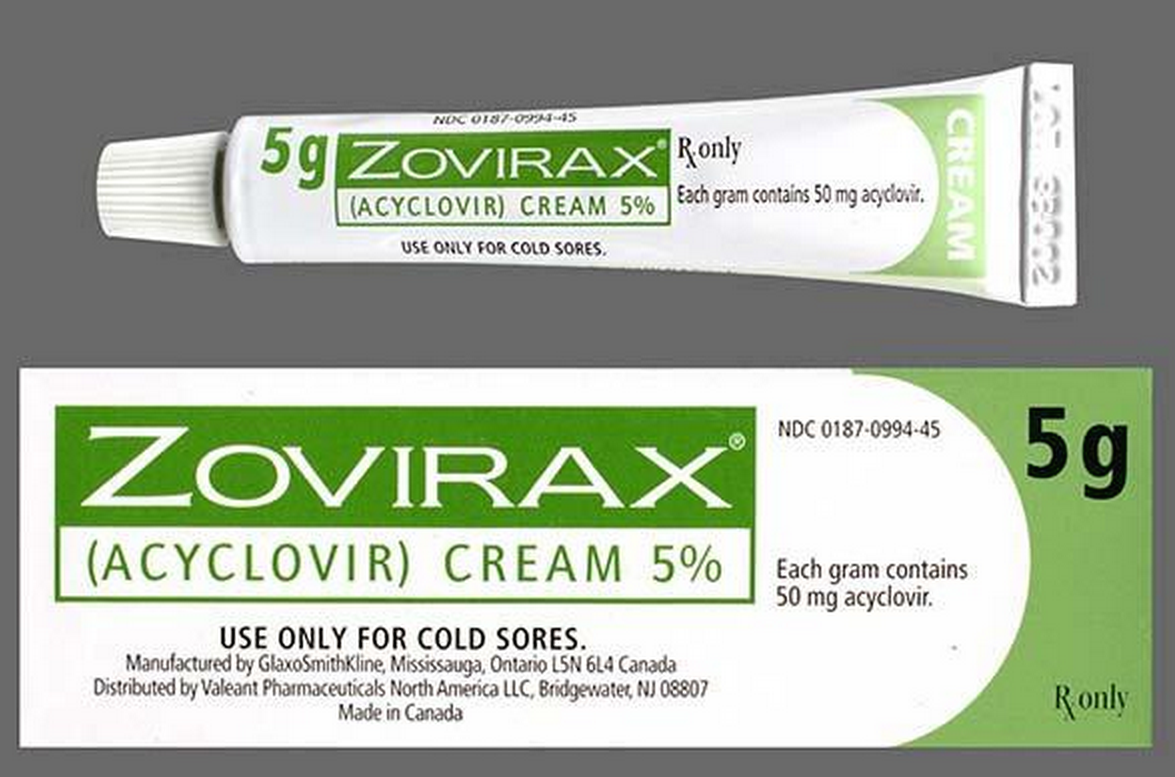
Perrigo Company plc has announced it has received final approval from the U.S. Food and Drug Administration for its AB rated Abbreviated New Drug Application referencing Zovirax® Cream, 5% (acyclovir cream, 5%) developed in collaboration with Sol-Gel Technologies Ltd. The Company anticipates launching the product this month.
Annual market sales for the twelve months ending December 2018 were approximately $92 million as measured by IQVIA™.
Perrigo Executive Vice President and President Rx Pharmaceuticals Sharon Kochan stated, "This final approval and first to market launch is another example of our long-term investment in new products, the team's impressive execution skills and our solid collaboration with SolGel. We stay committed to providing affordable treatment options for patients in important extended topical categories."




