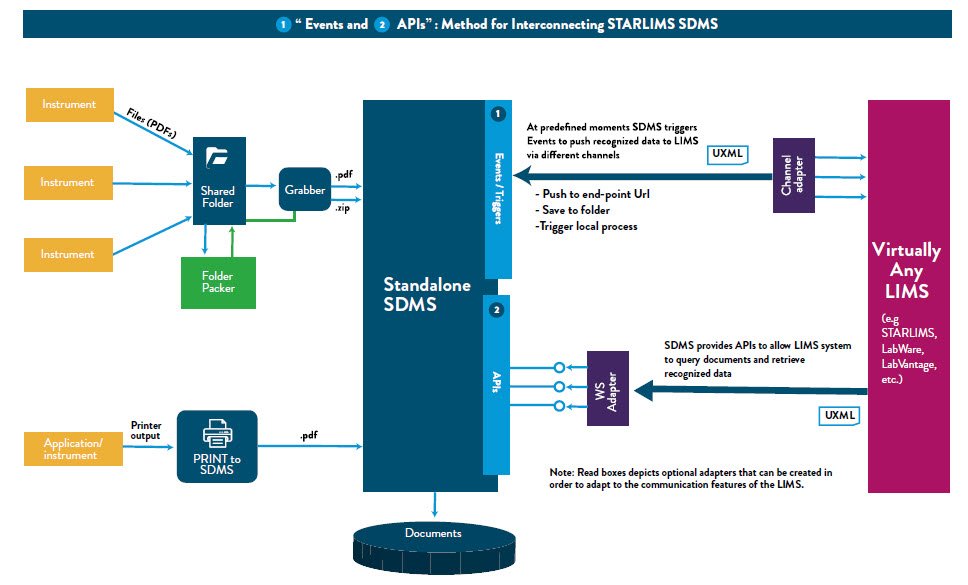
In addition to being available as part of the Abbott Informatics STARLIMS integrated solution, it is now available as a standalone product to help clients achieve compliance with the data integrity expectations of the Food and Drug Administration (FDA) as well as other regulatory authorities. STARLIMS SDMS V12.2 can work with an existing LIMS or without a LIMS, interfacing effectively with any LIMS (Laboratory Information Management System), CDS (Chromatography Data System), ELN (Electronic Laboratory Notebook), SAP (Systems Applications Products) and other lab systems through webservices without replacing them.
STARLIMS SDMS V12.2 can benefit three types of clients:
Abbott Informatics designed this solution specifically to help organizations comply with 21CFR Part 11 regulations; to ensure compliant record creation, audit trails, electronic signatures and data security and to offer the following benefits to clients:
STARLIMS Standalone SDMS can work independently or with an integrated LIMS system to achieve data integrity by capturing data in real time, backing up data, protecting it from deterioration and loss, and maintaining the raw data as the original record. All these benefits will help clients maintain quality control, minimize errors and meet regulatory requirements.




