BioSpectrum Asia Top 20 Survey Rank 9 - Dr. Reddy's Laboratories, India

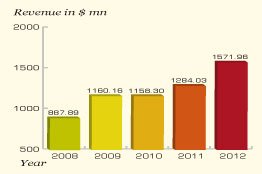
Striking profit on a high note, Dr. Reddy's crossed $1.5 billion worth of business in 2012, marking a growth of 22 percent. Dr. Reddy's generic version of fondaparinux, used for prophylaxis, treatment of deep vein thrombosis and acute pulmonary embolism, delivered a promising success and witnessed a surge in the US market.
Overall, Dr. Reddy's made a strong impression on the US market by launching Olanzapine 20 mg tablets, the generic version of the brand Zyprexa, used to treat schizophrenia and bipolar disorder. Olanzapine has added around $100 million to Dr Reddy's revenue for in 2012.
Dr. Reddy's continued to embark on its biosimilars business, clocked $26 million, and achieved 45 percent growth over last year. Until date, Dr. Reddy's has developed a strong market base for filgrastim, peg-filgrastim, rituximab and darbepoetin alfa for cancer in 13 countries in the emerging markets. So far Dr. Reddy's is supplying its biosimilar products to emerging countries and is receiving impressive response, given affordable price of the drug. The company is now planning to launch biosimilars in developed markets as well.
Giving higher emphasis on R&D in generics products, Dr. Reddy's hiked investment in R&D by 17 percent and allocated $125 million in FY2012. Considering the lucrative opportunities in Japanese pharmaceutical market, which is currently valued at $97 billion, Dr Reddy's forayed into the generic space in partnership with Fujifilm to develop, manufacture and promote generics drugs, and plans to launch its product in the next three to four years.
In the year 2012, Dr. Reddy's was aggressive in expanding its global footprint. Dr. Reddy's acquired 51.33 percent interest in Reddy Kunshan, a joint venture in China, engaged in manufacturing and marketing of active pharmaceutical ingredients and intermediates and formulations in China. The company is also leveraging on its newly acquired GSK's penicillin manufacturing facilities from GSK and product rights for Augmentin (branded and generic) and Amoxil brands in the US.




