image credit- shutterstock
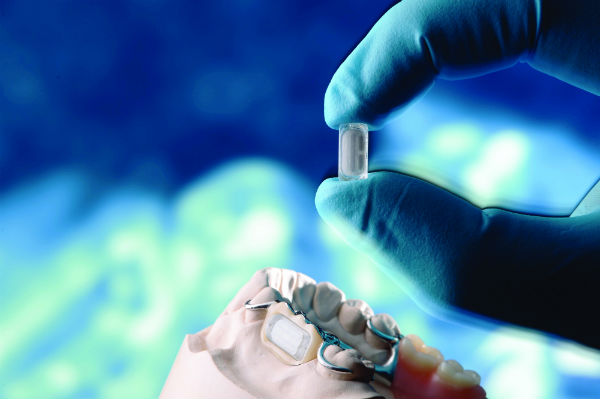
On August 30, 2021, Zimmer Biomet, a global medical technology leader from the USA with revenue worth $7.836 billion, and Canary Medical, a medical data company from Canada started rolling out the world’s first United States Food and Drug Administration (USFDA) approved smart knee implant, Persona IQ.
Once surgically implanted in the knee, Persona IQ records and wirelessly transmits a wide range of gait data to a patient's personal base station at home. The data are then securely delivered to a cloud-based platform. Surgeons can assess post-surgery recovery progress by comparing pre-operative mobility metrics captured by the my mobility app, with post-operative gait metrics collected by Persona IQ.
Canary’s foundational insight is that implanted devices sit at the site of a patient’s illness, and so can provide unique and invaluable data on device function and patient health. Yet, for most devices, when the last suture is closed, they go dark. Canary’s technology platform has already broken new ground in orthopaedics, creating the world’s first commercial talking knee, with hip and shoulder prostheses under development, with more applications soon to come in spine, trauma, aesthetics and vascular
Talking about the company’s plans Dr Bill Hunter, CEO, Canary Medical, Canada said “What Canary is doing in orthopaedics it aims to do in each of its targeted therapeutic areas: providing real-time collection, and near the real-time remote analysis of data that Canary Medical believes will be key to the next frontier of monitoring, diagnostics, and treatment.”
Stryker, another leading player in the space from the USA and Zimmet’s competitor, is also trying to enter this space with recent acquisitions. In January 2021, Stryker acquired OrthoSensor, a leader in the digital evolution of musculoskeletal care and sensor technology for total joint replacement.
Intelligent Implants is a Swedish startup developing the SmartFuse System to improve outcomes in lumbar spinal fusion procedures. In May 2021, the firm received FDA breakthrough device designation for SmartFuse.
Researchers at the USA-based University of Pittsburgh Swanson School of Engineering are creating patient-specific 3D-printed smart metamaterial implants that double as sensors to monitor spinal healing.
Researchers from around the world are also focused on developing smart implants. Researchers from Australia’s national science agency, The Commonwealth Scientific and Industrial Research Organisation (CSIRO) have developed new implantable devices equipped with machine learning to help prevent seizures and monitor patients after brain surgery. The researchers will now use a $1 million Australian Government grant awarded to Australian company Anatomics to develop a ‘smart helmet’ to monitor brain swelling in stroke and traumatic brain injury patients.
Medical device companies are increasingly trying to put sensors in the device so that it functions as theranostics - providing therapeutics and diagnosis for a disease before it can actually occur. The most widely implanted smart devices are the pacemaker/defibrillator and the cochlear implant. Smart medical implants are increasingly being used for both long-term and short-term post-operative monitoring. By ensuring rapid implementation of the necessary treatments, the implanted device enables early detection of adverse events, minimising post-operative complications.
Market opportunities
The global smart implants market is estimated at $3.9 billion in 2022 and is forecast to surpass a market value of $22.2 billion by 2032, according to Fact.MR report. The market is enormously compelling, with opportunities to create better outcomes for patients, better practice operations for clinicians, and cost savings for insurers.
Sharing his views on the market potential, Edward Yager, Head of Regional Strategic Marketing, Zimmer Biomet Asia Pacific said “In Asia, there is also a larger opportunity for those patients that live a considerable distance from their physician. If a patient lives one hour away, for example, that patient has a total travel time of two hours to see their physician for a follow-up. Plus, the patient may need a family member – daughter, son, partner – to travel with her/him which could include that family member taking time away from school or work. Now with smart implants, a patient and physician can be connected so that the physician has access to data about the patient’s activity level and recovery no matter the distance. There is also an advantage for when the patient and physician do meet, as there is a considerable increase in objective data to inform the physician or physician’s team on the progress being made by the patient. There are many win-win scenarios.”
But does the rising popularity and developments of smart implants mean they will be as ubiquitous as wearables?
“It seems likely that the trend will continue to grow and expand rapidly in the future as our society continues to become more aware and interested in increasing activities of daily living after surgery and returning to a more active lifestyle. Smart implants offer opportunities to not only improve/repair physical pain associated with osteoarthritis, for example, but also the opportunity for patients, their physicians, and the physicians’ care teams to understand more about the mental anxieties or concerns a patient may have regarding their recovery post-operatively and can act or answer questions more quickly because of the access to objective data on the patient’s recovery,” added Yager.
Dr Hunter, however, feels that the comparison is rather unfair because most wearables are fundamentally consumer products, not healthcare devices.
Security issues
Cybersecurity is a pressing issue for smart devices, especially healthcare devices. There’s an ongoing debate on the safety and how best to regulate these devices.
“Canary’s operating philosophy is that patients own their data; Canary is only a steward of that data, and always in the patient's interests. Our operating model is designed around those principles. For example, the raw data from canturio te, our tibial extension sensor technology, is encrypted and transmitted, via a base station located in the patient’s home, to Canary’s The Health Insurance Portability and Accountability Act (HIPAA) compliant, closed, cloud platform, where it is processed and then securely distributed to clinical and patient interfaces, which are regulated by the FDA. These data are only ever used in ways that patients have consented to, and patients can withdraw that consent whenever they wish,” said Dr Hunter.
Smart implant companies are using various security methods. These use encryption and unique code identifiers to communicate with one another. This can be similar to BlueTooth technology where two devices share a connection that is encrypted and unique to the two devices communicating.
“Data is anonymised so that there is no identifiable patient health information made available to third parties without patient consent. And companies work closely with countries’ health regulatory bodies to comply with local codes, laws, and regulations,” said Yager.
We are on the cusp of transformative breakthroughs in smart implants. When integrated with the traditional healthcare system, smart implants have the potential for massive cost savings and improved patient care.
Ayesha Siddiqui
ayesha.siddiqui@mmactiv.com




