Masimo and Mindray announce expanded partnership
13 May 2019 | News
Mindray Offer Masimo SET® Pulse Oximetry under its Patient Monitoring Devices in many Countries beyond the United States
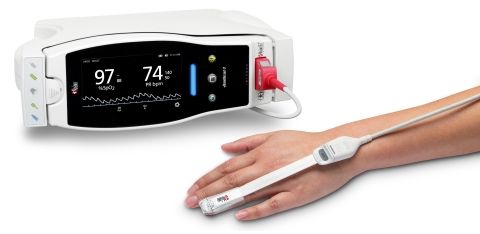
Image Courtesy: Businesswire
Masimo and Shenzhen Mindray Bio-Medical Electronics Co., Ltd have entered into a new purchase and license agreement. The new partnership, under Mindray, will offer Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry – noninvasive, continuous measurement of oxygen saturation (SpO2), pulse rate (PR), and perfusion index (Pi) in its monitoring devices. Mindray devices equipped with SET® will now be available in selected countries in Europe, the Middle East, Russia and the Commonwealth of the Independent States, and Asia-Pacific (excluding China), including Australia and India. Thus, the benefits of Masimo SET® are now also available to clinicians using Mindray’s devices in many countries outside the United States.
The new partnership has made advanced medical technology in healthcare more accessible by achieving excellent patient monitoring. Patients, clinicians, and hospitals can benefit from the performance of Masimo SET® pulse oximetry which includes the Mindray BeneVision N and BeneView T series for use in high-acuity environments and the ePM, iPM, and iMEC series for use in a variety of clinical scenarios, among other devices.
Mindray being a leading global provider of medical devices and solutions represents the world’s third-largest patient monitoring market share. With the invention of Signal Extraction Technology® (SET®), Masimo established a new standard for pulse oximetry by introducing the ability to measure through motion and low perfusion. SET® has demonstrated the highest sensitivity and specificity in identifying desaturation events and avoiding false desaturation events during these conditions.
SET® has also opened up new frontiers in patient monitoring during challenging conditions. Studies have shown that clinical assessment assisted by SET® helps clinicians reduce retinopathy of prematurity (ROP) in neonates, improve critical congenital heart disease (CCHD) screening in newborns, and through continuous monitoring of patients in post-surgical wards, reduce ICU transfers and rapid response team activations. Over 100 independent and objective studies have shown that SET® outperforms other pulse oximetry technologies. Masimo continues to refine SET® and recently announced that SpO2 accuracy specifications have now improved to 1.5% in conditions of motion and no motion for adult, pediatric, and infant patients (> 3 kg) with RD SET™ sensors.
Jon Coleman, President of Worldwide Sales, Professional Services, and Medical Affairs, Masimo, said: “We’re excited to enter into this agreement with Mindray, so that more patients, clinicians, and hospitals can benefit from the unmatched performance of Masimo SET® pulse oximetry.”
Yang Ting, General Manager of International Sales and Marketing, Patient Monitoring and Life Support, Mindray, said, “We are very happy to expand our cooperation with Masimo from North America to more regions so that our customers will have access to the outstanding SpO2 technology from Masimo. It is proof of our constant commitment to bringing advanced medical technologies to people in need, and making better healthcare more accessible for all.”












