Hope floats for HIV patients!
14 January 2014 | Analysis | By Rahul Koul Koul
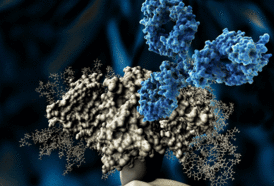
Neutralizing Antibodies bind to the four identified sites on the virus. (Pic Credits: IAVI)
Compared to the times of complete despair and loss of hope, there has been a remarkable scale up of treatment over the period of time, for HIV that causes Acquired Immunodeficiency Syndrome (AIDS) in the human body and its subsequent break down. However, the improvements have hardly helped to solve the deeper problem as it continues to require lifetime treatment, besides every one person put on treatment, two newly infected getting added to the list. The disease has been an area of major concern for the various global health agencies across the world, since its identification in 1983. The virus has already killed 35 million people globally. In India alone, there are around 2.7 million people who have been affected by the AIDS. Therefore, a broadly effective AIDS vaccine would be a powerful asset to the efforts to stop the spread of HIV. However, given the complexities in the nature of the virus, the forging of partnerships involving international collaborations and the open sharing of scientific knowledge, is expected to provide direction and boost to translational research.
Globally efforts continue
As per Dr Wayne C Koff, chief scientific officer & senior vice president - R&D, International AIDS Vaccine Initiative (IAVI), "Earlier we were not able to identify the proteins clearly. In last few years, we have been able to make the crystal structure. This has been made possible only because of advances in basic sciences. Evolution of the bNAb's in our times has led to understanding and raised new hopes. Combination of antibodies is important for tackling virus. Year after year, we have much more to talk about hopefully!"
Despite some failed attempts in past, the outcomes have been unable to deter the researcher's pursuit. "Though the science is the game of hits and misses, it is also a great learning experience where we learn from each failed effort to launch renewed efforts to tackle the situation. At the moment, we have identified four vulnerable sites on the virus and are now looking for more sites on the one found in India," said Dr Koff while responding to query on failure of few vaccine candidates in the past.
Founded in 1996, the IAVI is a global not-for-profit organization, whose mission is to ensure the development of safe, effective, accessible, preventive HIV vaccines to use throughout the world. It is associated with 25 countries to research, design and develop AIDS vaccine candidates. The HIV Vaccine Design Program will capitalize on recent research advances that have sparked a renaissance in AIDS vaccine research and development (R&D). In September 2009, scientists at IAVI and their colleagues in the Neutralizing Antibody Consortium (NAC), which is run by IAVI, reported the isolation of a pair of potent. And very broadly neutralizing antibodies against HIV, that discovery, the first of its kind in a decade, was followed by the isolation of other broadly neutralizing antibodies (bNAbs) by researchers at the Vaccine Research Center of the US National Institutes of Health and of still more by the IAVI-affiliated team. The most promising of these antibodies are now being scrutinized by researchers to elucidate the specific mechanisms by which they bind to and neutralize HIV. The idea is to create artificially synthesized mimics of their targets on HIV, to be used in vaccines to elicit similarly potent bNAbs and teach the immune system how to repel HIV infection.
Dr Robin A Weiss, emeritus professor, University College London, chair - IAVI Scientific Advisory Committee and HVTR Laboratory Scientific Advisory Group, said, "From being a death sentence, the HIV-AIDS has now been called a treatable disease from the year 1996 onwards. People with HIV have worst cases of tuberculosis (TB). With 60 percent of them being affected, the women are the worst sufferers in the most affected nations such as Africa."
Playing a responsible role
The Indian government on its part has been playing a very active role. Apart from health ministries, National AIDS Control Organization (NACO) that has been advocating on preventive measures and social awareness, the various other agencies from science and technology ministry have encouraged research in the area. The Pune based National AIDS Research Institute (NARI) funded by Indian Council for Medical Research (ICMR) has progressively expanded its activities in various aspects of research on HIV and AIDS through infrastructural development, capacity building and research programs. Another big initiative is Gurgaon based, The Translational Health Sciences and Technology Institute (THSTI), an autonomous institute of the Indian government's Department of Biotechnology (DBT) which will primarily focus on one of the greatest scientific challenges of AIDS vaccine design and development. In collaboration with the IAVI, the program is focused on the elicitation of antibodies capable of neutralizing a broad spectrum of circulating HIV variants, a problem that stems in large part from the almost unparalleled mutability of HIV.
The IAVI-THSTI collaborative program is participating in a much coordinated, global effort to create replicas of bNAb targets in the laboratory for use as immunogens, which are the active ingredients of vaccines. This program has been charged with the complex task of developing, testing and then implementing strategies to rapidly screen large numbers of bNAb-based immunogens against HIV-1 and to prioritize them for further evaluation in preclinical studies. It is expected that the program using high throughput (HT) screening will ultimately lead to strategies for next generation immunogen design and expand the number of AIDS vaccine candidates available for assessment in human trial.
Dr Rajat Goyal, country director - India, International AIDS Vaccine Initiative (IAVI), mentions that its aim was never the ownership. "We were happy when the government of India chipped in with the funding. Pushing the clinical research towards the drug development was our focus and the DBT with its work on research projects, Indian Council for Medical Research (ICMR) on clinical aspect and NACO on the epidemiological part have been key partners." He also feels great about the amount of funding from DBT and ICMR, having been scaled up in the recent years.
The THSTI-IAVI program is an integral part of the THSTI cluster of research centers. It will be linked closely to both the hub of the NAC, the IAVI Neutralizing Antibody Center at The Scripps Research Institute in La Jolla, California, and to IAVI's AIDS Vaccine Design and Development Laboratory in New York. The work conducted also complements a current partnership IAVI has with the Indian Medicinal Chemistry Program (IMCP), under the auspices of the DBT, to design and generate conceptually novel HIV immunogens. Other institutions participating in this partnership include the International Centre for Genetic Engineering and Biotechnology in New Delhi, and the Indian Institute of Science, Bangalore.
According to Dr Sudhanshu Vrati, head, Vaccine & Infectious Disease Research Centre, and dean, Translational Health Science and Technology Institute (THSTI), other than funding that has come from IAVI, they have looked at the technical expertise. "We just completed phase III of rotavirus vaccine. The new opportunities in vaccines have arrived and increase in knowledge base. We look forward to applying the same in this case as well."
The partnership has collateral benefits for the nation that goes beyond the advancing of development of broadly effective AIDS vaccines. Most significantly, the DBT expects that it will contribute to India's efforts to become a world leader in translational research-the science of converting promising laboratory concepts into biomedical products that can be tested in people and, ultimately, manufactured by industry.
The annual operational funding for the program as per Dr Sukhdev Sinha, advisor, DBT, is close to Rs 40 crore. The DBT has put another Rs 6 crore into various 25 projects that are into R&D of these diseases. He adds, "India holds a very unique place in the vaccine development. We know that the vaccine development success stories in India have made us to believe in wonders. Now the focus is more on few major disease areas such as HCV, HIV and TB. At the moment, along with the DBT, the DST, CSIR and other too are putting money in HIV infection research in one or the other way."












